With us, tissue is not an issue
Let’s find out whyWe are a clinical research organization and develop life science tools
To help you research more effortlessly with 3D human tissue technology
The EHT¹ workflow
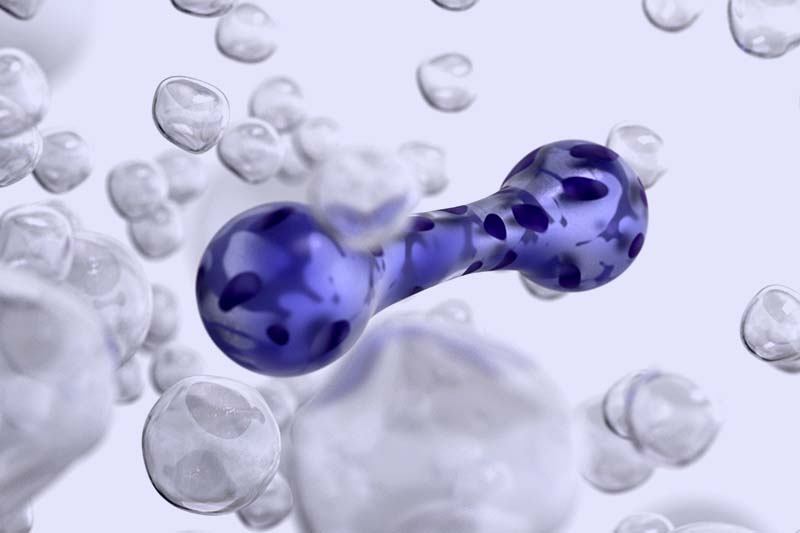
EHTs, created from human induced pluripotent stem cells, are 3D cardiac constructs that accelerate preclinical research and enhance personalized medicine studies.
Manual tissue analysis is a thing of the past. That’s a promise.
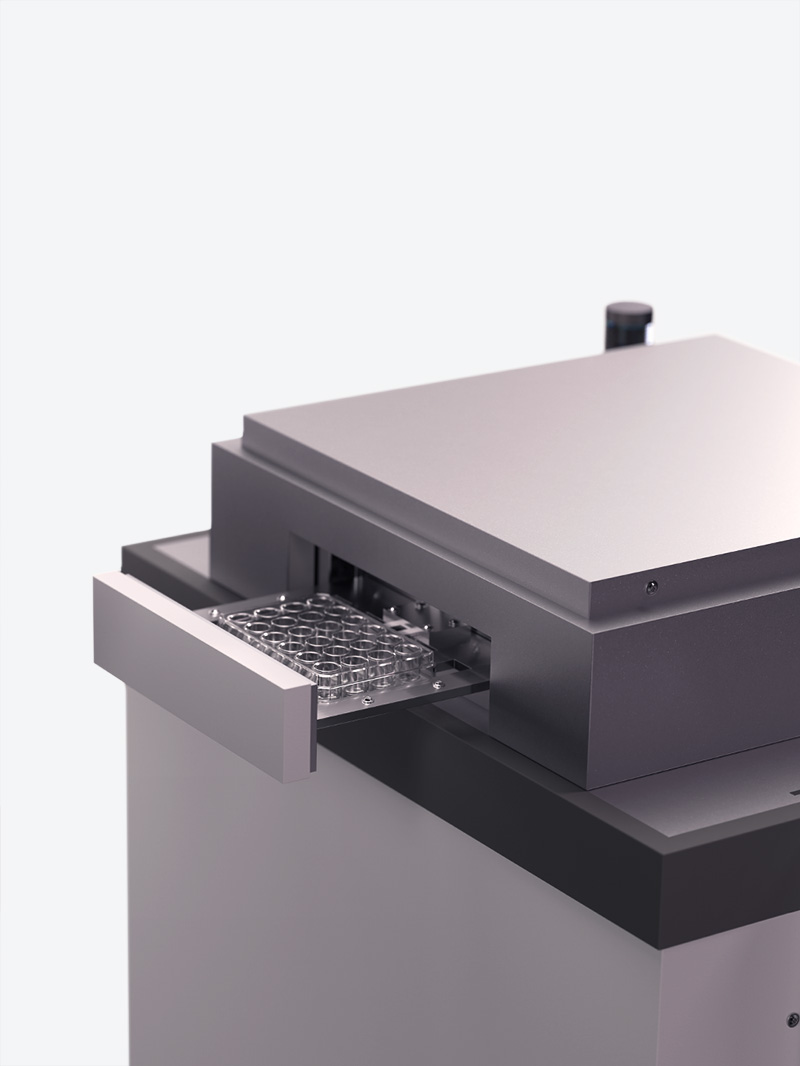
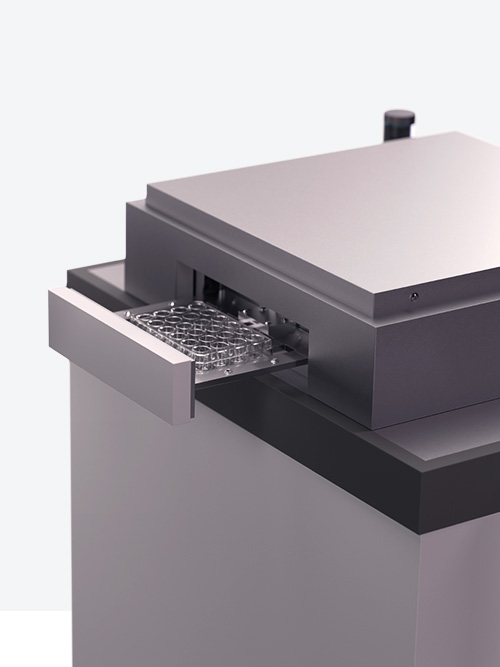
02Run sterile tests in 90 s
02Run sterile tests in 90 s
With the DiNAQUBE™, our AI-driven measurement platform, research has never been more effortless. In just 90 seconds, you can run sterile, video-optical measurements that allow for long-term, non-invasive studies.
Capable of parallel analysis, the DiNAQUBE™ can simultaneously analyze the contractions of up to 24 Engineered Heart Tissues (EHTs), ensuring that you capture all essential time-dependent effects. And, due to our automated tissue recognition technology, you can rely on accurate, user-independent results every time.
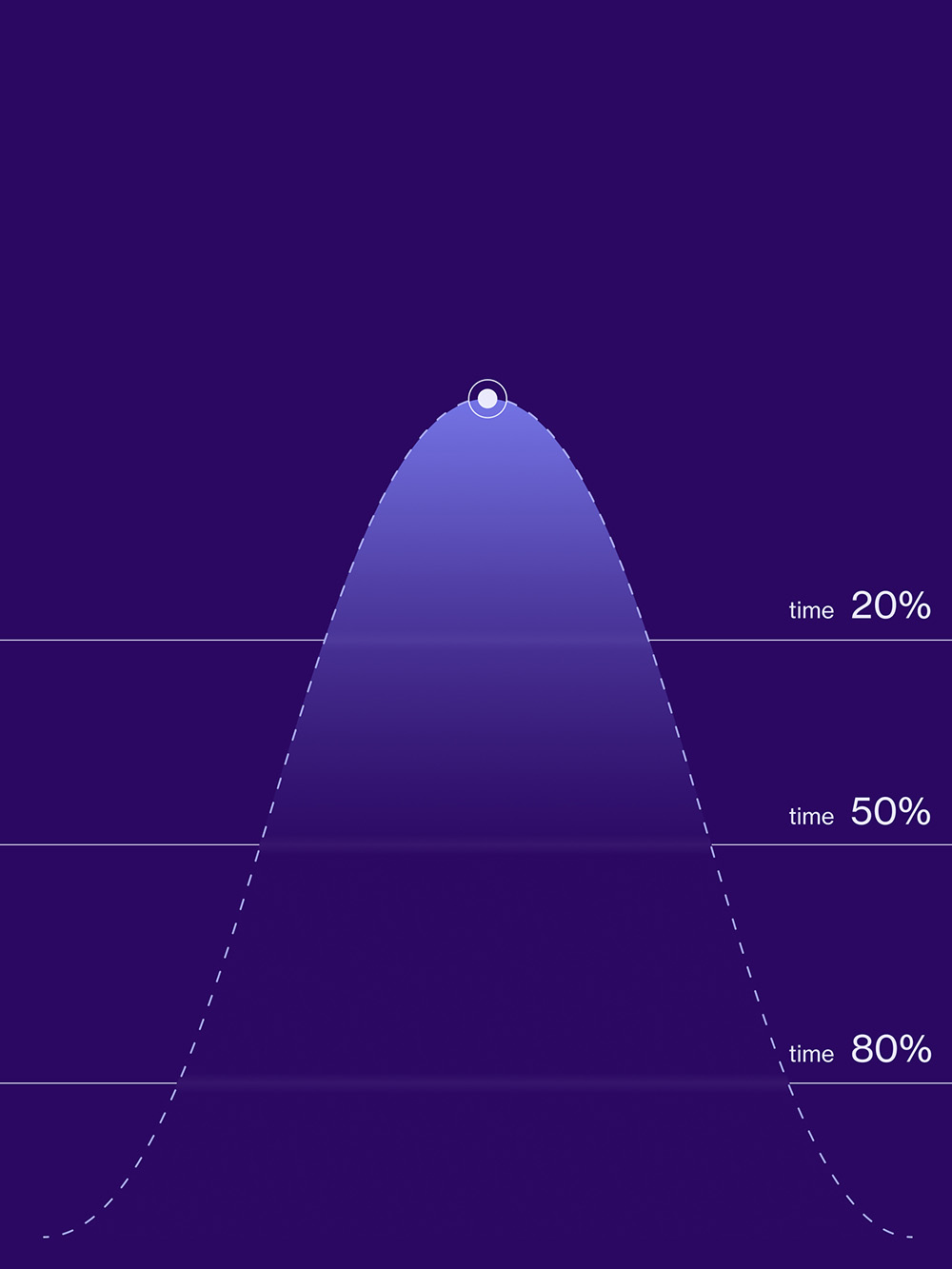
03Interpret ready-to-use data
03Interpret ready-to-use data
With our web-based data analytics solution, designed for ease of access and use, you can effectively retrieve and analyze human tissue data measured with our DiNAQUBE™. Features such as customizable reporting, automated quality control through artificial intelligence, and integration with multiple data sources allow you to focus more on data interpretation and less on data management.
In addition, machine learning capabilities simplify and accelerate the classification and comparison of measurements, providing valuable insights and ensuring consistency in data quality and analysis.
About us
Trust those with a legacy in leading edge technology

You choose how we serve you best
Your team’s extension or as in-house platform
from our lab
Services for early-stage drug development
in your lab
The DiNAQUBE™